NP Thyroid Recall by Acella
Thyroid news report:
On May 22nd, 2020 Acella Pharmaceuticals issued a voluntary nationwide recall of certain lots of NP thyroid due to super potency.
Don’t worry, I’m going to explain in plain English what this all means and how it applies to you.
If you aren’t already aware, NP thyroid is a prescription thyroid medication that falls into the class of medications known as NDT or natural desiccated thyroid.
NDT medications contain a combination of both T4 and T3 thyroid hormones whereas most other thyroid medications, like levothyroxine, contain only T4 thyroid hormone.
This recall was issued for only SPECIFIC lots of NP thyroid and it does NOT apply to all of the doses that they offer.
What this means is that certain lots (which I will explain more below) were incorrectly adjusted in regards to their dose and they ended up with more thyroid hormone than they were supposed to.
This super potency problem applies ONLY to specific lots (13 of them) and ONLY to 30mg, 60mg, and 90mg NP thyroid tablets.
DOWNLOAD FREE RESOURCES
Foods to Avoid if you Have Thyroid Problems:
I’ve found that these 10 foods cause the most problems for thyroid patients. Learn which foods you should avoid if you have thyroid disease of any type.
The Complete List of Thyroid Lab tests:
The list includes optimal ranges, normal ranges, and the complete list of tests you need to diagnose and manage thyroid disease correctly!
What is Superpotency?
Super potency is a word used to describe how powerful something is.
When using this term in regard to thyroid medication, we are talking about how strong any given tablet of thyroid medication is.
All formulations of NDT thyroid (including NP Thyroid) are controlled and regulated to have a very specific amount of thyroid hormone in each grain.
For NP thyroid that magic number is 38mcg of T4 and 9mcg of T3 in each and every ‘grain’ (which is a unit of measurement of thyroid medication).
This particular issue only affects the amount of T3 in each formulation of NDT.
So instead of having 38mcg of T4 and 9 mcg of T3 in each grain of NP Thyroid the medication is superpotent because it contains 115% of T3 or 15% more than what it is supposed to have (and what the label shows that it has).
Put into real numbers, this means each grain of NP Thyroid of this lot has 38mcg of T4 and 10.35mcg of T3 (instead of the regular 9 mcg).
You can see that this small change really isn’t anything to write home about nor is it likely to cause any problems but we will talk more about that below.
The lots affected by this super potency problem are listed below:
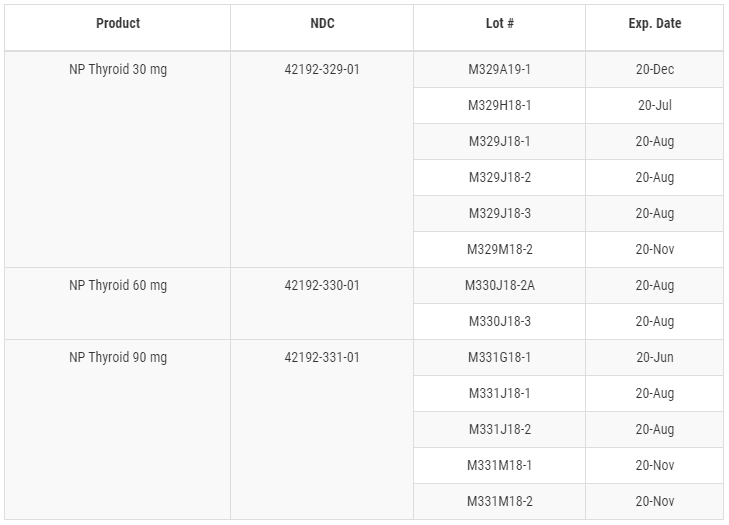
- M329A19-1
- M329H18-1
- M329J18-1
- M329J18-2
- M329J18-3
- M329M18-2
- M330J18-2A
- M330J18-3
- M331G18-1
- M331J18-1
- M331J18-2
- M331M18-1
- M331M18-2
What are lots?
Lots are just a way to describe a large group of medications (but this also applies to supplements) that are created under the same circumstances including ingredients, doses, etc.
Placing your medications into lots allows you to keep an eye on manufacturing and helps you manage problems as they arise.
Without these lot numbers, NP thyroid would have to potentially recall every single dose of medication that they suspect has problems.
It’s also important to note that if your medication does NOT fall into one of these lots then you do not have anything to worry about.
How do you find your lot number?
You should be able to find the lot number of your medication, usually abbreviated “Lot No.:” near the expiration date like the picture below:

What Should you do if your lot is affected?
What should you do if you find out that your lot of NP Thyroid is affected by this super potency issue?
The standard thing you will hear is to go back to your doctor to get the medication swapped out for a lot that isn’t a problem.
And I would agree that this is probably the best course of action for most people.
Why?
Because there is a very small risk (emphasis on very small) that taking these super potent doses may cause hyperthyroid symptoms.
But it’s also worth pointing out that the risk of this happening is INCREDIBLY small.
Remember, there’s only a 1mcg difference in T3 between the superpotent dose and the regular regulated dose.
In reality, this small change in dose is not likely to cause any changes to how you feel except it may actually help you to feel better (because most people do better on more T3).
If possible, though, you would want to swap it out for a non superpotent lot simply because it may impact your thyroid lab tests and cause confusion for you and your doctor as you try to figure out what doses will work for you.
If, on the other hand, other lots of NP thyroid are NOT available to you (due to backorders, pharmacy stock problems, etc.) there’s a strong argument that it would be better to stay on the super potent dose as opposed to swapping over to something like levothyroxine.
I would be very hesitant if your doctor insists that you switch medications because of this recall.
This is only an isolated one-time problem and staying on the superpotent dose would likely be better for your body than swapping medications entirely, especially to T4-only thyroid medication.
The False Belief that NDT isn’t Regulated
Even though this is a less-than-ideal thing to happen, especially to NDT medications which already have a bad reputation among endocrinologists, there is some silver lining.
One of the main tools or reasons that doctors insist on not prescribing NDT formulations such as Armour thyroid and NP thyroid is that they believe that these medications are not regulated.
A common talking point that you will hear from doctors goes something like this:
“NDT comes from a pig and is not regulated so you don’t know how much thyroid medication you are getting when you take it.”
This statement, in all its forms, is used as a justification to NOT prescribe these types of medications even though they can seriously benefit and help many people.
This recall, while a terrible thing, actually proves that this point is not true.
If it were true that these medications were not regulated and contained various amounts of thyroid hormone in them there would be no reason for Acella to recall the medication.
And this recall shows that they ARE regulated and each formulation is supposed to contain a specific amount of thyroid hormone in each grain of NDT.
So this recall can be used by you, as a thyroid patient, as a tool to help your doctor understand that this common belief is absolutely wrong.
Your Next Steps
What should you do if you are taking NP thyroid?
Check the lot number on your medication bottle and determine if you are taking a lot that is affected.
If you are, you can call your pharmacy or your doctor’s office and they can help you with getting a new prescription with a non superpotent lot.
Now I want to hear from you:
Are you currently taking NP Thyroid?
Are you taking an affected superpotent lot number?
If so, have you noticed any changes in your symptoms?
How are you handling this recall?
Leave your comments or questions below!








